RECOMMENDATION FOR COSMETIC INDUSTRY OF SAFETY
Its a reality that reinforces the importance and reach of the personal care industry. The European cosmetics industry trade association.

Real Talk About Talc A Cosmetic Chemist Gives It To Us Straight Affordable Skin Care Natural Skincare Packaging Chemist
The responsible person is required to keep the PIF on file for 10 years.

. A Guide to United States Cosmetic Products Compliance Requirements. And beauty makers like Avon are donating soap to help countries around the world limit the spread of COVID-19. The FDAs Voluntary Cosmetic Registration Program VCRP is a reporting system for use by manufacturers packers and distributors of cosmetics that.
Food and Drug Administration CFSAN Outreach and Information Center 5001 Campus Drive HFS-009 College Park MD 20740-3835 1-888-SAFEFOOD 1-888-723-3366. Provided they are used in accordance with the manufacturers instructions there. Cosmetics Manufacturing Industry Regulations.
We make the following recommendations. Public consultation will begin in early 2022. When it comes to cosmetics product safety is one of the biggest concern of either legal administrations consumers or manufacturers.
CIR - Cosmetic Ingredient Review - This is an independent group of scientists regulators funded by the cosmetic industry which is responsible for evaluating the safety of cosmetic ingredients. It also determines brands credibility and product quality. However the recommendations of this review may be relevant to the work of Ahpra and relevant National Boards in the cosmetics sector more widely.
SAFETY OF COSMETIC PRODUCTS 271 of FDA authority over the cosmetic industry it is important to understand the breadth of its authority and that cosmetics in the marketplace are not operating in a vacuum without oversight relative to safety. Cosmetic Product Safety Report. Specifically the FDA has the authority to.
From the knowledgeable experts of the Consumer Product Safety Commission CPSC Environmental Protection Agency EPA Food and Drug Administration FDA Federal Trade. Under this regulation cosmetic manufacturers cannot misbrand products and must gain approval of all color pigments Ref. Personal Protective Equipment PPE.
Indeed manufacturers have the responsibility of ensuring that each product sold is safe for consumers. The California Safe Cosmetic Act aims to protect the health of consumers by requiring cosmetic manufacturers importers or distributors whose business volumes are over 1 million to report the ingredients contained in the cosmetic. Licensees should wear masks as a safety measure when providing a service.
If chemical or biological in-teractions between the ingredients exist the evaluation to the generated risk substances shall be performed. Skincare was the leading category accounting for. Such identification is not intended to imply recommendation or endorsement by.
The PIF contains the quality efficacy and safety information for cosmetics and cosmetic ingredients manufactured in or imported into the EU. Having been formed over 50 years ago the association has had its fair share of putting sanity in the cosmetics industry. The primary focus will be on cosmetic surgery because that poses the greatest risk.
We look forward to working with the Minister for Patient Safety and the Department for Health and Social Care to improve the landscape surrounding aesthetic non-surgical cosmetic treatments for the benefit of the industry and public safety and hope that these recommendations will be carefully considered and acted upon. Read the full Terms of Reference here PDF 32KB. Colipa is the European trade association for the cosmetic and personal care industry.
The association has come up with guidelines that each stakeholder must conform to in order to run in the industry. Disposable masks should be made available to patron and may only be used for a single customer. With the understanding that PPE is often not worn properly will be in very short supply moving forward and will likely become very costly.
Cosmetic Products Safety Composition and Labelling Cosmetic Products Safety Regulations 2008. Licensees should wear masks as a safety measure when providing a service. These masks can be disposable or cloth and must be disposed of or washed properly as required by the CDC.
The Food Drug and Cosmetic Act of 1938 currently governs the cosmetic manufacturing industry Ref. The CPSR forms the foundation of the Product Information File PIF. The batch number of manufacture or the reference for identifying the goods.
A report required to be prepared by the responsible person the person linked to a cosmetic product in th EU as defined by Article 101 of European Council regulation 12232009. Ous ingredients the safety of ingredients is the premise of cosmetic safe-ty. However corporations have long treated animals inhumanely in the name of science to test products on animals.
17 Chemicals and product safety All products should be safe to use as cosmetics but they can have occupational health effects so it is important to obtain the material safety data sheets MSDS for all products used. The cosmetic industry being such an intimate product with the consumer requires high health regulation to ensure product safety. From this one can easily derive or draw conclusions regarding the safety of a cosmetic product.
The FDA loosely regulates the cosmetic industry. JOINT COLIPAEWF RECOMMENDATION SAFETY OF PETROLATUM AS RAW MATERIAL FOR THE COSMETIC INDUSTRY Background For the purpose of their carcinogenicity classification in the Dangerous Substances Directive 67548EEC all petroleum substances were allocated to a number of distinct groups according to their refinery processing history. 22 The cosmetic safety evaluation shall be based on.
The safety evaluation of cosmetics shall be based on the risk assess-ment of all ingredients and risky substance. Ban or restrict ingredients due to safety concerns. These masks can be disposable or cloth.
ASEAN Guidelines for safety evaluation of cosmetic products Page 3 of 15 OBJECTIVE. The purpose of this Guideline is to help the Cosmetic Industry in assessing the safety of the product as well as the Regulators in auditing the data contained in the Product Information File PIF. The report needs to cover safety information and a safety asessment including an explanation of the scientific reasoning.
If you are wondering about the safe levels of ingredients that are used in the cosmetic industry then this is the place to find the information. Skincare hair care make-up perfumes toiletries and deodorants and oral cosmetics are the main product categories of the cosmetic market. In fact after avoiding exposure handwashing is the CDCs top recommendation for people to protect themselves from COVID-19.
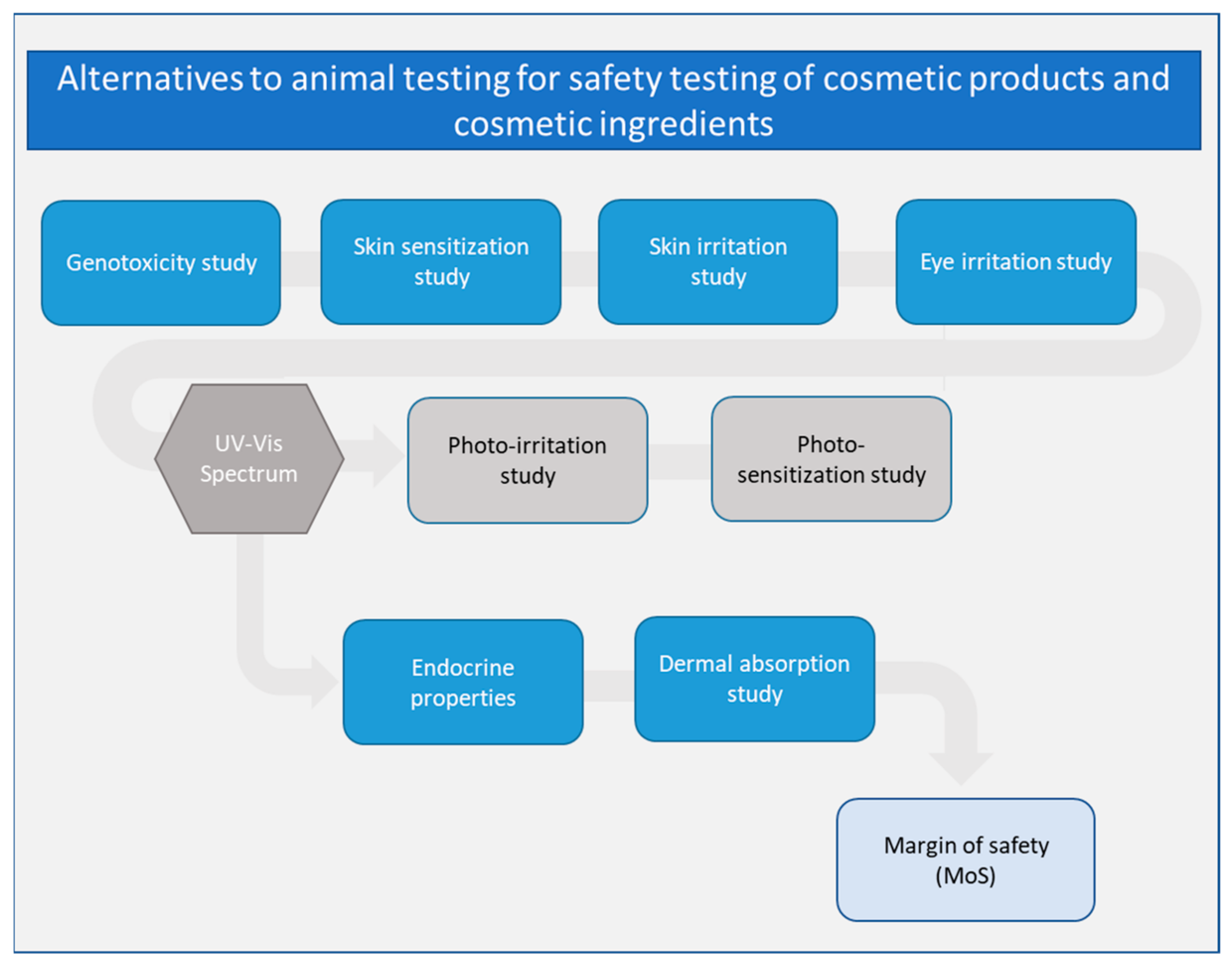
Cosmetics Free Full Text Safety Testing Of Cosmetic Products Overview Of Established Methods And New Approach Methodologies Nams Html

When I Learned The Truth About The Dishonesty In The Beauty Industry I Had To Make A Change So Thankfu Beautycounter Inspirational Quotes Safe Beauty Products
Cosmetics Europe The Personal Care Association You Your Products

Cosmetic Industry Requirements Regarding Skin Models For Cosmetic Testing Sciencedirect

A Step Forward On Sustainability In The Cosmetics Industry A Review Sciencedirect

Article David Suzuki Fdn Safe Cosmetics Body Care Lead In Lipstick

Formulations In Cosmetic And Personal Care De Gruyter T Https Www Amazon Co Uk Dp 3110452367 Ref Cm Sw R Pi Dp In Cosmetics De Gruyter Making Cosmetics

Belum ada Komentar untuk "RECOMMENDATION FOR COSMETIC INDUSTRY OF SAFETY"
Posting Komentar